Schambony Lab – Research
Development of any sexually reproducing organism begins with a single fertilized egg. In vertebrates, this spherical fertilized egg, the zygote, develops into a multicellular embryo with a distinct shape that already has formed the primordia of tissues and organs. During this phase, the three germ layers form during gastrulation, cells migrate within the embryo and specific tissues develop and acquire their typical shape, a process that is called morphogenesis. We investigate how shape changes in the early embryo are regulated by biochemical signals, mainly by beta-catenin independent Wnt signaling pathways.
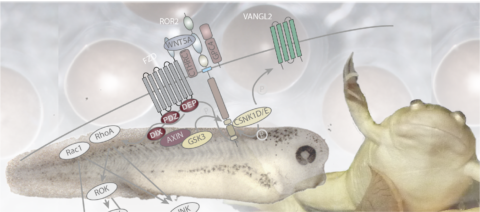
Wnt signaling pathways are activated by Wnt-family extracellular glycoproteins. Notably, Wnt pathways have been identified in animals from cnidarians to mammals. Since the first description of a Wnt pathway, several Wnt-stimulated intracellular signaling cascades have been characterized. To date, the major distinction is made between beta-catenin dependent and independent pathways. Among the latter, the so-called Wnt-PCP pathway is a major regulator of cell polarity within planar tissues and plays an important role in coordinating cells during morphogenesis in early vertebrate embryos. In the adult, Wnt signaling has been linked to the progression and metastasis of multiple types of cancer and is therefore of major interest in biomedical research.
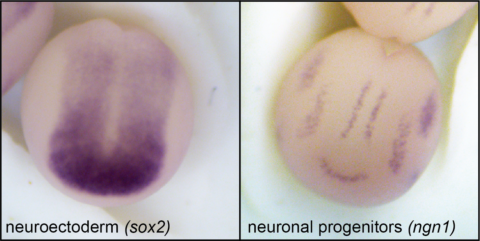
With increasing cell numbers and progressing cellular differentiation, the biochemical landscape such as cell surface proteins and extracellular biochemical signals changes and guides further development. In parallel, the mechanical landscape such as surface tension, fluctuations and tissue stiffness also changes, however knowledge about the impact of mechanical changes as instructive cues in early development is still scarce. As part of CRC1540 – Exploring Brain Mechanics, we aim to investigate the cross-talk between biochemical signals and mechanical factors in early nervous system development in the model organism Xenopus laevis.
Molecular interactions in Wnt/β-catenin signaling
Influence of substrate stiffness on early neural development
Research